RU58841 is applied topically, where it acts to block DHT from affecting the hair follicles. Because RU58841 only affects the scalp, it has practically no side effects. It is highly effective in stopping the progression of androgenic alopecia. It does not, however, significantly stimulate hair re-growth and is, therefore, most effective if applied early in the balding process. In combination with minoxidil to stimulate hair regrowth, it may actually be the first truly effective cure for balding.
Male Pattern Baldness
Androgenic alopecia, more commonly known as male pattern baldness, affects millions of men and women worldwide. In androgenic alopecia, individuals inherit hair follicles that are particularly responsive to a hormone called dihydrotestosterone (DHT). DHT is a hormone derived from testosterone that plays an important role in maintaining male sexual characteristics. However, in individuals who inherit androgenic alopecia, DHT also causes hair follicles to undergo a process called miniaturization, during which they cease to produce normal hair. Eventually, baldness occurs.
A variety of treatments have been introduced for androgenic alopecia, but none can be said to be “the cure.” Several oral drugs that block the conversion of testosterone to DHT have been introduced (e.g., finasteride) that stop the progression of androgenic alopecia, but these drugs can have significant sexual side effects. In addition, they do not seem to work on women with androgenic alopecia. Topical minoxidil can help stimulate re-growth of hair, but it is only partially effective in most people.
Where Did RU58841 Come From?
RU58841 was first synthesized in France in the 1990s as part of experiments to identify anti-androgen drugs. It was found to be an effective anti-androgen useful for treating various hormone-related conditions such as acne, excess hair growth, and hair loss. Several studies in animals models demonstrated it was quite effective in treating androgenic alopecia (1, 2). In 2008, the rights to RU58841 were purchased by Proskelia Pharmaceuticals, who re-named it PSK3841. However, for various reasons such as the fact that the drug cannot be patented (patent expired), no pharmaceutical company has invested the necessary money and time in getting the drug approved for sale to the general population to treat androgenic alopecia. A few small human clinical trials of the safety of the drug for topical use have supposedly been conducted, but none have yet been published (3).
Structure of RU58841
RU58841 is a non-steroidal anti-androgen drug. It is an arylhydantoin with a bicyclic-1H-isoindole-1,3-(2H)-dione structure that is similar to RU58642 and nilutamide. Nilutamide is approved for use in treating prostate cancer (4). It is an oral medication that blocks androgens from binding to and stimulating the growth of prostate cancer cells. RU58642 was synthesized as part of the same series of experiments that included synthesis of RU58841. At the time of its synthesis, RU58642 was thought to be one of the most potent anti-androgen drugs ever discovered (5). However, very little has been done with RU58642 since its discovery. It is occasionally used in laboratory studies of the androgen receptor.

The non-steroidal hydantoin anti-androgen drugs are considered preferable to the older steroidal anti-androgen drugs because the hydantoin drugs exhibit little to no cross-reactivity with other hormone receptors. Many of the steroidal anti-androgen drugs cross-react with estrogen or progesterone receptors, causing unwanted side effects. They also have poor bioavailability and tend to be toxic to the liver. Hydantoins in general have excellent bioavailability, being readily absorbed through either the skin or the digestive tract.
RU58841: How it Works?
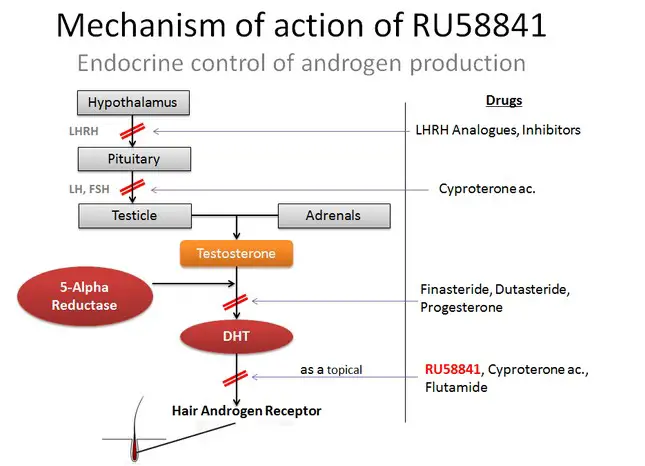
The androgen receptor is a protein that is expressed at high levels in the prostate, skeletal muscle, liver, and epididymis. It is also expressed in the dermal papilla at the base of hair follicles. The androgen receptor exists primarily inside the cytoplasm of the cell. When an androgen (testosterone or DHT) binds to the androgen receptor, it moves into the nucleus of the cell, where it affects the regulation of gene expression. Anti-androgen drugs act by binding to the androgen receptor (6). However, after these drugs bind to the receptor, it does not move into the nucleus. Instead, it remains in the cytoplasm, bound to the anti-androgen drug. Since the receptor is blocked by the drug, it cannot bind to any androgen molecules, and it is therefore inactivated. The antagonist effect of RU58841 on androgen receptors can be summarized below:
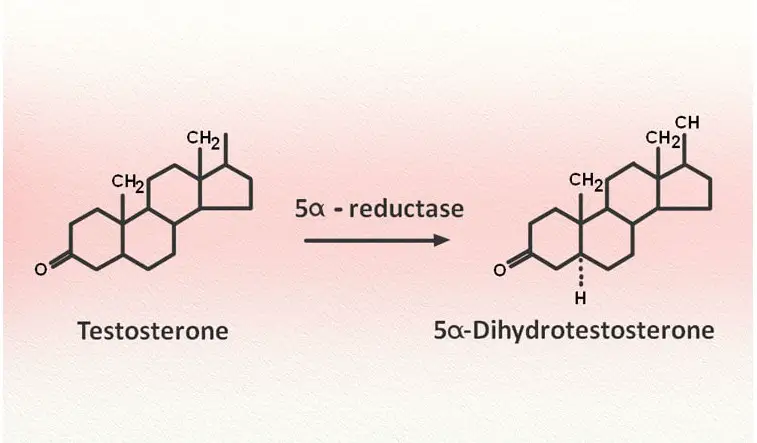
Testosterone is a steroid that is primarily synthesized in the testicles. It is also synthesized in small amounts in the ovaries and the adrenal glands. It is released into the bloodstream and taken up by cells throughout the body. In some cells, it is converted into DHT by the enzyme 5-alpha-reductase. DHT is a much more potent androgen than testosterone, binding to the androgen receptor with much greater affinity. The androgens play important roles in maintaining secondary male sexual characteristics, sexual drive and function, bone health, and blood cell formation. When referring to their reproductive effects, the term “androgenic effects” is used; when referring to their effect on muscle and bone, the term “anabolic effect” is used.
When anti-androgen drugs such as nilutamide are taken orally, they block androgen receptors throughout the body, down-regulating both androgenic and anabolic effects of androgens. The side effects can be fairly significant. For example, nilutamide commonly causes decreased libido, impotence, and visual side effects. When RU58841 is applied to the scalp, it is able to penetrate the skin and enter the cells of the hair follicles (7). However, it does not enter the bloodstream and therefore has no systemic effects. The only cells that have their androgen receptors blocked are those in the local area. Clinical studies in monkeys and rats were unable to identify any systemic side effects after applying RU58841 to the skin (1, 7). Volunteer users of RU58841 report minimal side effects, either.
Why Isn’t RU58841 Available At the Pharmacy?
When people first hear about RU58841, most are immediately skeptical. Their first thought is that if some pharmaceutical company had actually found a miracle cure for baldness, the company would be heavily marketing it. It would have been pushed through FDA approval and would be widely available from doctors and at pharmacies. The pharmaceutical company would be making vast amounts of money. After all, 70% of men and 40% of women are affected by androgenic alopecia. That’s a lot of customers.
RU58841 History
RU58841 was first studied in the 1990s by scientists in France, working for the Roussel-UCLAF Corporation. Initial studies on the compound reported amazing success in treating hair loss in animal models without any side effects. However, in 1995, a huge German company called Hoechst absorbed the entire Roussel-UCLAF company. Hoechst had originally purchased a significant proportion of Roussel-UCLAF back in 1968. By 1994, Hoechst had obtained over 50% of Roussel-UCLAF’s shares and proceeded to negotiate with the company to buy the rest of it. The Supervisory Board of Roussel-UCLAF agreed to the merger.
HMR Group
Following the absorption, Hoescht and Roussel-UCLAF became part of the Hoescht-Marion-Roussel (HMR) Group, composed of a merger between Hoescht, the German company, and the company Marion Merrel Dow, a US company, and of course Roussel-UCLAF, the French pharmaceutical company. As of 1995, HMR became a huge international company focused on pharmaceuticals, agriculture chemicals, and industrial chemicals.
Restructuring
During the restructuring process after the merger, many employees of Hoescht and Roussel-UCLAF lost their jobs. Numerous research projects that Roussel-UCLAF had been working on were simply abandoned. The executives running the new HMR apparently believed that only three areas of pharmaceuticals are profitable, namely drugs that target disorders of the central nervous system, drugs that target cardiovascular disease, and drugs that fight bacterial and viral infections. This misguided view of the ailments of human biology led to the simple abandonment of many promising projects in the areas of endocrinology and immunology, including RU58841.
Outrage
Employees and scientists of Roussel-UCLAF were reportedly quite shocked by the new policies. The bizarre decision to abandon entire areas of research meant that promising treatments for breast cancer, cerebral edema, cirrhosis of the liver, addiction, acne, and, of course, baldness, were simply dumped. At least 65 research projects and 169 pharmaceuticals with great potential to improve human health and quality of life were abandoned for no real reason other than that some executives thought they might not prove profitable enough.
Abortion Pill
One of the more famous drugs that was dumped during this merger is RU 486, also called mifepristone, the abortion pill. At the time of the creation of HMR, Roussel-UCLAF was manufacturing and selling large amounts of the drug worldwide. After the merger, HMR donated the rights to the drug in the US to the Population Council and to a company called Exelgyn for all non-US uses. Currently, mifepristone is widely used in safe, effective abortions and is also used in emergency contraception. The reason(s) why HMR would be willing to discard such a widely-used and profitable drug is unclear. They may be in part related to religious beliefs held by the then-head of Hoescht. However, the primary reason is the odd decision by the new executives of HMR to simply abandon all endocrinology research under the assumption that endocrinology isn’t profitable enough to bother with.
Rights Transfer
After the merger, the rights to RU58841 were acquired by a company called ProStrakan. ProStrakan may have changed its name first to HMR-3841 and then to PSK-3841. The company apparently performed some limited research with the drug, including a human trial that was never published. Although the results of the trial were reported to be quite encouraging, the company appears to have abandoned all future research and development of the drug. The rationale for this may be a Morgan Stanley report prepared in 2005. The analyst who wrote the report concluded that the market for the drug was “modest” and therefore it would only be of interest for a small specialized dermatology company. ProStrakan is a fairly large pharmaceutical company that currently markets drugs for pain, nausea, osteoporosis, and cancer. Apparently it simply isn’t interested in developing and marketing a drug that only has a “modest” potential market.
Current Legal Situation
Currently, RU58841 can be legally manufactured and sold as a research chemical by anyone who wants to engage in the practice. Because no one can patent the drug, there is little reason to believe that any pharmaceutical company will want to expend the necessary funds in order to develop the drug and get it approved by the FDA and other regulatory agencies. The profit margin simply isn’t there. The miracle cure for baldness, that belongs on the pharmacy’s shelf, has been abandoned in the pursuit of profits. This not an unusual situation by any means- the World Health Organization estimates that only 37% of known human diseases are being targeted by pharmaceutical companies, entirely due to perceptions about possible profits.
Ru58841 Side Effect
RU58841 is applied to the scalp, where it is taken up by the hair follicles. In theory, it could then enter the bloodstream and affect other parts of the body. Studies in monkeys did not detect any systemic RU58841 side effects from topical applications (1). However, some individuals who have tried RU58841 claim they experienced systemic RU58841 side effects. The side effects reported include skin irritation, decreased libido, erectile dysfunction, strange sensations, red eyes, blurred vision, nausea, aches and pains, dizziness, and headaches (2).
In Theory
The skin irritation seems to be entirely due to the carrier used to dissolve the RU58842; some users use too much ethanol or other innately skin-toxic substances for this purpose (propylene glycol…). Individuals who use other carriers do not report any skin irritation. It is unclear if the other RU58841 side effects are actually due to the RU58841 or are just a placebo effect. As an androgen blocker, it is theoretically possible for systemic RU58841 to cause reduced libido and erectile dysfunction. Nilutamide, an androgen blocker used orally to treat prostate cancer, is reported to occasionally cause blurred vision, dizziness, and aches and pains (3).
Mathematical Model
However, a mathematical model of the breakdown of RU58841 clearly demonstrates that even if it is absorbed efficiently into the bloodstream, its active metabolites can never achieve a dose high enough to cause any anti-androgenic side effects (4). The model is based on knowledge about the breakdown of RU58841. After application to the scalp, the original RU58841 molecule has a half-life of only around 1 hour. It primarily breaks down into RU59416, a compound that only weakly binds to androgen receptors. Around 1% of RU58841 breaks down into RU56279, also called cyanonilutamide. Cyanonilutamide has powerful anti-androgenic effects. It is similar in structure to anti-adrogenic drugs used to treat prostate cancer, and if it were to enter the body in a high enough dose, it would be expected to cause side effects similar to those reported for nilutamide (3).
Cyanonilutamide
Cyanonilutamide has a half-life of about 20 hours in the body. This long half-life has caused some to suggest that regular use of RU58841 can cause cyanonilutamide to build up in the body over time, eventually reaching a dose that causes side effects. However, the mathematical model mentioned above has proven this does not happen. The formula to estimate the decay of a drug over time is based on the basic mathematical formula for exponential decay:
This basic formula needs to be modified to take into account application of a new dose of RU58841 each day:
If you begin calculating from the day of the very first application of RU58841 this formula is simplified to a geometric series :
Does Not Build Up
Assuming a daily dose of 100 mg of RU58841 is applied, and also assuming that all of the cyanonilutamide produced enters the bloodstream (unlikely), a dose of 1 mg of cyanonilutamide is being taken each day. Rounding the 20 hour half-life of cyanonilutamide up to 24 hours to simplify the calculations and plugging these numbers, you end up with:
This formula shows us that the amount of cyanonilutamide in the body cannot go over 2 mg, even if you use RU58841 daily for years.
Too Low
Two mg of cyanonilutamide is a miniscule dose that is almost certainly incapable of causing any noticeable side effects. Therapeutic doses of other androgen blockers that are given orally are in the 150 to 750 mg range. However, a 2 mg dose isn’t going to block very many androgen receptors. It therefore seems very unlikely that any of the RU58841 side effects are actually caused by the RU58841.
RU58841 Results
Before after pics found in differents hairloss forum through the web (baldtruthtalk, hairloss talk, hairloss help etc) from many RU58841 users (thanks to them for the follow-up):
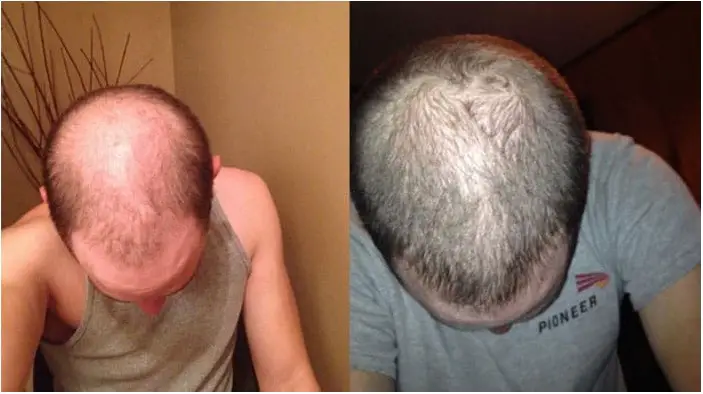
You can add your results or share your experience by leaving a comment below
Preparing RU58841 For Safe Use
If you’ve decided to join the many men who are fighting hair loss with RU58841, the next step is to prepare the agent for use.
Selecting a Carrier
Pure RU58841 is sold as a white powder. It has to be dissolved in a solvent before being applied to the scalp. Most men have the best results when they combine RU58841 treatments with minoxidil treatments. Therefore, one alternative is to use a solution of minoxidil to dissolve the RU58841. The combined solution allows for an easy one-step application. Others prefer to dissolve the RU58841 in a special solvent called K&B Solutions Carrier, and then either apply the minoxidil separately or forego its use altogether. Remember that K&B Solutions Carrier should be refrigerated after receipt. Solutions of RU58841 are generally stable for about 6 months after preparation.
Mixing with K&B Solutions Carrier
To prepare a 5% solution of RU58841, buy a 50 ml bottle of the solvent. Measure out 2.5 grams of RU58841 powder and pour into the bottle and shake as described above.
Mixing with Minoxidil
Purchase a 60 ml bottle of minoxidil. To make a 5% solution, measure out 3 grams of RU58841 powder and pour into the minoxidil using a spoon, funnel, or paper (see below). Shake the bottle gently for about 5 minutes until the powder completely dissolves.
Measuring RU58841
RU58841 is sold as a white powder in various amounts. The most accurate way to measure RU58841 is to purchase a kitchen scale. Alternatively, you can simply purchase only the exact quantity of powder that you need for your chosen recipe. For example, if you are planning to mix 3 grams into 60 ml of minoxidil, purchase a 3-gram package. If you’d rather buy a larger package and only use part of it, you can use common sense to estimate the proper amount of powder to use. For example, if your recipe calls for 2.5 grams and you have a 5- gram package, just use half of the powder.
Transferring RU58841
In laboratories, powders are often weighed on a small square of paper that is then creased or folded to form a funnel to allow careful transfer of all of the powder into the solvent. A small piece of waxed paper can be used at home for this purpose. Alternatively, a clean, dry spoon or small plastic funnel can be used to carefully transfer all of the powder into the solvent.
Premade Solution
Many ask, why bother mixing up your own solution when you can purchase pre-made solutions? Some people do have success with the pre-mixed solutions, but with the pre-mixed solutions it is buyer beware. The purity, quality, and concentration of the RU58841 is not guaranteed. Many pre-mixed solutions do not even indicate what solvent has been used to prepare the solution or how fresh the solution is.
RU58841 Result / Success
Once you begin using RU58841 regularly, keep in mind that results will not be seen immediately. It usually takes 4 to 6 months for RU58841 to stop hair loss. If minoxidil is also being used to help stimulate hair regrowth, again, it takes 4 to 6 months for noticeable results to occur. Regular use of a quality, fresh product at the correct concentration will yield results, just not overnight.
COMPARISON WITH FINASTERIDE
Androgenic alopecia, or male-pattern baldness, is mediated by the action of the hormone dihydrotestosterone (DHT) on the hair follicles. Hair follicles express androgen receptors in the cells of the dermal papilla. Individuals who inherit a predisposition to androgenic alopecia seem to express higher levels of androgen receptors in the dermal papilla (1). The hormone DHT binds to the androgen receptors and stimulates the hair follicle to spend more time resting and less time growing hair. Over time, repeated exposure to DHT causes the hair follicle to undergo a process called miniaturization, after which the follicle only produces tiny, barely noticeable hairs called vellum.
5-alpha-reductase blockers
Since the process of balding is mediated by DHT, researchers speculated that blocking the production of or the action of DHT may prevent, stop, and possibly even reverse the balding process. DHT is manufactured in the tissues from testosterone through the action of an enzyme called 5-alpha-reductase. A number of drugs that block the production of DHT have been developed to treat benign prostatic hyperplasia. One such 5-alpha-reductase blocker, called finasteride, has been approved by the FDA to treat androgenic alopecia.
Finasteride (trade name: Propecia)
Finasteride is an oral medication. It has been proven in clinical trials to be somewhat effective in treating baldness in men; however, it does not appear to work in women affected by androgenic alopecia (2). Around 70% of men who take finasteride stop losing hair. A tiny minority experiences some hair regrowth. The drug has to be taken continuously because the effect rapidly goes away as soon as the drug is discontinued. As a systemic treatment, finasteride decreases the amount of DHT throughout the entire body, not just in the scalp. As such, it can cause sexual side effects such as decreased libido and erectile dysfunction, and these side effects may be permanent even after the drug is discontinued. It may also increase the risk of certain types of aggressive prostate cancer and male breast cancer (3). It can cause fetal defects if pregnant women are accidentally exposed to it. Also, some men report feeling depressed and “foggy” while taking the drug.
Dutasteride
Dutasteride is similar to finasteride in that it blocks the creation of DHT. Dutasteride is a more potent drug than finasteride because it blocks all three forms of the 5-alpha-reductase enzyme whereas finasteride only blocks two of the forms, and finasteride has a much shorter half-life in the body. Dutasteride is primarily used to treat benign prostatic hyperplasia. Although not approved by the FDA for balding, it has been used off-label to treat androgenic alopecia. It has been shown to be slightly more effective than finasteride for stopping balding and stimulating the re-growth of hair (4). However, it has the same systemic side effects as finasteride, except they seem to occur even more frequently because dutasteride significantly lowers serum levels of DHT relative to finasteride.
RU58841
Unlike finasteride, RU-58841 does not affect production of DHT. Instead, it binds to the androgen receptors and blocks them from interacting with DHT. Blocking DHT prevents the hormone from causing the balding process. Preliminary studies suggest that RU58841 is at least as effective as finasteride in stopping balding, and it seems to be much more effective than finasteride in promoting hair re-growth, particularly if used in combination with minoxidil. However, the real advantage of RU58841 is the lack of systemic side effects. It is only applied to the scalp. It does not enter the systemic circulation, and it does not affect hormonal levels in the body. As such, it does not appear to have any side effects at all (5).
RU58841 versus Finasteride/ Dutasteride
All three of these agents are quite effective in stopping the balding process. RU58841 may be more effective than finasteride or Topical dutasteride for re-growing hair. However, finasteride and dutasteride have systemic side effects that may be unacceptable to most men, particularly men in their 20s and 30s who are beginning to experience balding and want to stop the process as soon as possible. RU58841 does not appear to have any systemic side effects.
How Does RU58841 Work To Treat Baldness?
Each hair grows out of a follicle that is embedded in the skin. The hair itself is not alive, but the base of the hair follicle, called the papilla, is composed of very active cells. They divide faster than any other cells in the body, and therefore require a lot of nourishment, provided by a rich bed of tiny blood vessels that surround the papilla. The cells in the papilla produce the complicated structure we call hair. Human scalp hair grows about 15 centimeters per year (1).
Hair growth occurs in cycles. Unlike most mammals that grow hair seasonally, human scalp hair growth cycles are essentially random. Most of the hair follicles at any given time are in the active growth phase, called anagen. Each hair follicle spends 2 to 6 years rapidly growing hair during the anagen phase. At some point, however, each hair follicle enters a phase called catagen that lasts for about 3 weeks. After categen, the hair follicle enters a resting phase called telogen that normally lasts for up to 100 days. During this period of time, the hair produced by the follicle is usually shed. At any given time, around 8% of the hair follicles on the head are in telogen and are shedding hair. Shedding 25 to 100 hairs per day is normal for most people. After resting and shedding the old hair, each hair follicle re-enters anagen and begins to produce a new hair.
In people affected by androgenic alopecia, the presence of male hormones (DHT) causes the hair follicle to spend an excessive amount of time in telogen instead of quickly re-entering anagen. In addition, each time a hair follicle goes through telogen in the presence of DHT the hair follicle shrinks. After a few growth cycles, the shrunken hair follicle is only capable of producing tiny fine hairs called vellum. RU58841 blocks DHT from binding to the receptors in the hair follicles, allowing the hair follicle to re-enter anagen quickly after telogen. Blocking DHT also prevents the hair follicle from shrinking during telogen. In the presence of RU58841, the hair follicles function normally rather than spending excessive amounts of time resting and shrinking.
RU58841 was originally synthesized during experiments intended to discover a potent androgenic (male hormone) blocking agent (2). Many health conditions, everything from acne to prostate cancer, can be treated by blocking male hormones. However, administering androgenic blockers systemically causes a lot of severe side effects. For conditions only affecting the skin, if the blocking agent can be applied to just the skin, it can treat the problem locally without causing side effects.
Clinical studies in monkeys, who are affected by androgenic alopecia just like people, have shown that topical application of RU58841 completely blocks the balding process and also stimulates hair re-growth while baldness progression continued in the control group (see below) (3) .
Conculsion
To ensure that these results apply to people, an additional study using human skin affected by androgenic alopecia was also conducted and found similar results (4). Because the drug is applied to the skin, where it can easily penetrate the base of the hair follicles, it does not enter the systemic circulation. Because it is only acting locally, it has no systemic side effects. Human volunteers report that the application of RU58841 to the scalp completely stops the balding process after 4 to 6 months of regular use.
Once the hair follicles have been protected from DHT for an extended period of time, they seem to start to recover and begin to re-grow hair. Most volunteers also use minoxidil to encourage hair re-growth. Minoxidil improves blood circulation to the hair follicle, nourishing the cells as they grow hair. No systemic side effects have ever been reported in association with the application of RU58841 to the scalp.
- AI Powered Bald Filter Online 2024: See Yourself with No Hair! - January 19, 2024
- Harklinikken Bad Reviews 2024: Analyzing Negative Feedbacks - January 18, 2024
- How to Get the Alex Eubank Hair | Step-By-Step Tutorial 2024 - January 18, 2024